In recent years, laser-assisted laryngeal microsurgery has emerged as a valuable tool in the field of otorhinolaryngology surgery and is widely acknowledged as the gold standard treatment for vocal cord tumours [1]. Laser-assisted laryngeal microsurgery has the advantage of enabling precise targeted incision and resection of the musculomembranous vocal cords while ensuring shorter operation duration, less bleeding, reduced post-operative pain and minimal damage to adjacent nerves [2]. Patients who underwent laser microsurgery treatment reported favourable oncologic outcomes and a high level of laryngeal functional preservation while enabling early swallowing and maintaining voice quality [3].
Laser-assisted laryngeal microsurgery requires the maintenance of optimal anaesthesia depth in order to enhance muscle relaxation and ensure that the vocal cords remain stationary throughout the procedure. Hence, a critical aspect of laryngoscopic laser microsurgery success lies in the admi-nistration of neuromuscular blocking agents [4]. Mivacurium is a robust, short-acting, non-depolarising drug which provides the deep neuromuscular block necessary throughout laser-assisted microsurgery owing to intense intraoperative laryngeal stimulation [5]. Since mivacurium has the advantages of a short duration of action with no cumulative effects during continuous administration [6], it is theoretically a suitable choice for laser-assisted laryngeal microsurgery.
Mivacurium chloride, first synthesized in 1981, shares a structural resemblance with a benzylisoquinolinium compound known as atracurium and is primarily metabolized by plasma cholinesterase [7]. Mivacurium is composed of a combination of three isomers, namely the cis-trans isomer (36%), the trans-trans isomer (58%), and the cis-cis isomer (6%) [8]. It has an onset time of 170 seconds, a clinical duration of 16 minutes, and an effective dose causing 95% suppression of the twitch response (ED95) of 0.08 mg kg−1 [9, 10]. Mivacurium has an elimination half-life ranging from 2.6 to 3 minutes, and does not produce rapid tolerance in the body after prolonged infusion unless there is a deficiency in buty-ryl-cholinesterase [11]. It is a competitive antago-nist of acetylcholine at the postsynaptic nicotinic receptors and is hydrolysed by plasma cholinesterase enzymes [12]. It is noteworthy that acetylcholinesterase is incapable of hydrolysing mivacurium, and the in vitro hydrolysis rate of mivacurium correlates with the activity of butyrylcholinesterase [13, 14]. Mivacurium metabolites have no pharmacological activity, and are mainly excreted by the kidneys, with a very small portion excreted by the liver [15].
When mivacurium is administered to adults at doses ≥ 0.2 mg kg–1, some patients may experience histamine release leading to hypotension [12]. At elevated dosages, mivacurium has been associated with a temporary decrease in blood pressure, which is occasionally accompanied by facial flushing, typically lasting for a brief duration of 2 to 5 minutes [16]. Although the recovery from a mivacurium-induced neuromuscular block occurs quickly, the extensive use of neuromuscular blocking agents might result in residual muscle relaxation after surgery [17]. There was a strong correlation between patients’ early recovery after the administration of an initial dose of miva-curium and their subsequent complete recovery of muscle strength after discontinuation of a mivacurium infusion [18]. Huang et al. [19] administered an initial dose of 0.25 mg kg–1 of mivacurium, followed by a continuous infusion at 0.3 mg kg−1 h−1, and found that mivacurium cisatracurium besylate is more favourable than cisatracurium besylate in laser laryngeal microsurgery due to its less significant impact on haemodynamics, quicker postoperative recovery, absence of neuromuscular blockade accumulation and fewer adverse reactions [19]. In other types of surgery, the required infusion dose of mivacurium to maintain a 90–99% muscle fibrillation inhibition effect was in the range 3–15 μg kg−1 h−1 [11, 20, 21].
In clinical settings, myorelaxation monitoring involves the assessment of muscular responses through various electrical stimulation methods, such as the “train of four” (TOF), which consists of four separate stimulations (T1 to T4) with a 0.5-second interval applied to the ulnar nerve at the wrist [22]. In the past, it was reported that in-adequate reversal of residual block to achieve a TOF ratio > 0.7 was linked to an elevated risk of postope-rative pulmonary complications [23].
With the advent of acceleromyography, achieving a TOF ≥ 0.9 is essential to prevent residual neuro-muscular blockade and safeguard the airway from aspiration before tracheal extubation [24]. Lien et al. [18] recorded that the duration between the reappearance of the first and the third discernible twitch in the TOF count was 2.9 minutes.
In the present study, neuromuscular block was controlled with mivacurium via an automated closed-loop infusion pump. Closed-loop total intravenous anaesthesia is a technique which accurately adjusts drug infusion rates based on real-time monitoring of haemodynamic and anaesthetic depth variables while taking into account interindividual variations in physiological processes [25, 26]. In the past, neuromuscular block was controlled with mivacurium via a closed-loop controller (An Asena GH, Alaris Medical Systems, Basingstoke, Hampshire, UK) and the mean necessary mivacurium infusion rate was 7.0 ± 2.2 μg kg−1 [21]. Liu et al. [27]stated that the CONCERT-CL closed-loop infusion system effectively mitigates the pharmacokinetic constraints of target-controlled infusion (TCI) while maintaining the bispectral index within an ideal range. Closed-loop delivery performance of mivacurium achieved better control of neuromuscular block compared to atracurium, vecuronium, and rocuronium [28]. Pasin et al. [29] compared the efficacy and performance between BIS-guided closed-loop delivery and manual delivery, and concluded that closed-loop infusion maintains a better anaesthesia depth and reduces recovery time.
At present, the optimal infusion rate of mivacurium for maintaining the appropriate degree of muscle relaxation during laser-assisted laryngeal microsurgery is highly debatable. This study aimed to investigate the effects of different doses of mivacurium during anaesthesia in patients undergoing laser-assisted laryngeal microsurgery for vocal cord tumours using a concert-CL closed-loop neuromuscular blockade integrated TCI system. The benefits of determining the optimal infusion dosage of mivacurium include achieving optimal myorelaxation, avoiding overdose, preventing postoperative residual neuromuscular blockade, and mitigating potential side effects.
METHODS
Participants
A total of 67 patients diagnosed with vocal cord tumours scheduled for general anaesthesia-supported laser-assisted laryngeal microsurgery were recruited from the Department of Otolaryngology at the Fourth Hospital of Hebei Medical University between January 2015 and September 2015. This prospective randomized clinical trial adhered to the ethical principles of the World Medical Association Declaration of Helsinki [31]. The local medical ethics review committee of the Fourth Hospital of Hebei Medical University approved the patients’ selection for this study. This study was registered under the clinical trial registry of Hebei Medical University with registration number No. 2016031. All participants provided written informed consent.
Inclusion and exclusion criteria
The inclusion criteria for the study were as follows: patients aged between 18 and 65 years, of any gender, with American Society of Anesthesiologists (ASA) grade I–II and without neurological or muscular disorders. The exclusion criteria comprised patients with severe cardiopulmonary diseases, abnormal liver or kidney function, poorly controlled hypertension or diabetes, neuromuscular diseases, skin damage and a history of asthma and malignant arrhythmias. Individuals with previous surgery at the site of neuromuscular monitoring or who were administered medications affecting neuromuscular transmission within the past 3 months were excluded. Obese participants with body mass index (BMI) > 30 kg m–2 as well as pregnant or lactating women were also excluded. All patients who experienced adverse events such as a difficult airway, severe arrhythmias, hypotension, excessive bleeding, asthma, or severe allergic reactions during the procedure, which prevented the scheduled laser-assisted laryngeal microsurgery, were also excluded.
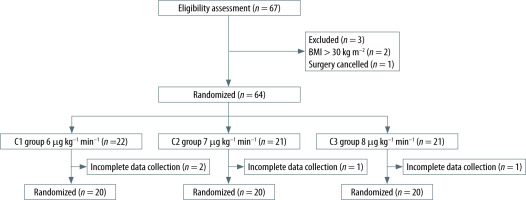
Two patients were excluded for having a BMI greater than 30 kg m–2, while 1 patient was excluded because the surgery was cancelled. In addition, 4 patients withdrew from the study due to incomplete intraoperative data collection. The final analysis was conducted based on the data collected from 60 patients (Figure 1).
Randomization
In this study, a randomized controlled trial design was adopted. Patients were assigned numbers based on the order of their treatment, and random numbers were generated by the statistical software SPSS. Subsequently, the enrolled patients were allocated to 3 equal groups, namely C1, C2 and C3, through randomization.
Sample size
The sample size for the present experiment was estimated using the Power Analysis and Sample Size (PASS) 15.0 software (NCSS LLC., Kaysville, Utah, USA). The test level a was set at 0.05, and the test power 1–β was 0.8. Participants were categorized into three groups in this study. Using the recovery index as the primary observation metric, the mean and standard deviation for each group were estimated based on both existing literature and a preliminary experiment. According to the software calculations, the minimum sample size required was 19 cases per group, leading to a total of 57 subjects. In order to account for potential dropouts during the trial, the decision was made to have 20 cases in each group, resulting in a total of 60 cases.
Anaesthesia methods
Once patients entered the operating theatre, a catheter for intravenous administration was esta-blished in the right hand and 0.9% sodium chloride was infused before induction. The patients’ vital signs, including blood pressure, heart rate (HR), blood oxygen saturation (SpO2), and bispectral index (BIS), were monitored. Oxygen was delivered with a flow rate of 4–6 L min–1 via a standard mask to provide oxygenation and facilitate nitrogen washout. Remifentanil (1.5 μg kg–1) and propofol (2.0 mg kg–1) were used to induce anaesthesia intravenously. Once the Modified Observer’s Alertness/Sedation scale (MOAA/S) score of patients reached zero, a target-controlled infusion of mivacurium was administered at a dose of 0.2 mg kg–1 in all groups. This standardized approach was implemented in order to ensure uniformity in the initial conditions across all groups, and satisfactory intubation conditions were achieved at TOF 0. After achieving maximum suppression at the first twitch (T1), an enhanced endotracheal tube was inserted under direct laryngoscopic guidance to secure the airway. Following intubation, mechanical ventilation was initiated using an anaesthesia machine in volume-controlled mode. The volume-controlled ventilation settings included a fresh gas flow rate of 2 L min–1, an FiO2 of 60%, a tidal volume of 6–8 mL kg–1 and a respiratory rate of 12–14 breaths/min to maintain EtCO2 levels in the range 35–45 mmHg. Anaesthesia maintenance was achieved through a continuous infusion of propofol at a concentration of 2.5–3.5 mg kg–1 and remifentanil at a rate of 0.2–0.3 μg kg–1 to maintain BIS values between 45 and 60. The infusion of these anaesthetic agents was halted at the end of the surgery. Mivacurium was administered using initial target-controlled infusions of 6 μg kg–1 min–1 in group C1, 7 μg kg–1 min–1 in group C2, and 8 μg kg–1 min–1 in group C3, with a dose escalation rate of 10 μg kg–1 min–1 when T1 recovered to 1%. When the TOF ratio was ≥ 0.9, the endotracheal tube was withdrawn and mivacurium was discontinued. If the patient’s HR decreased to < 50 beats min–1 during the procedure, 0.5 mg of atropine was administered. When the mean arterial pressure (MAP) dropped below 65 mmHg, ephedrine was admini-stered at a dose of 5–10 mg. In the event of significant adverse reactions such as pronounced erythema, bronchospasm, severe arrhythmias, or blood pressure fluctuations during the injection process, the trial was discontinued. A clinical criterion was used to determine the time of extubation by the anaes-thesiologist [30].
Neuromuscular monitoring and recording methods
After the patient entered the operating room, a closed-loop target-controlled infusion Willy Ark Concert-CL (Guangxi VERYARK Technology Co., Ltd, CHINA) was connected to the left upper forearm to monitor the neuromuscular conduction function and control the infusion rates. The TOF stimulation was applied at an interval of 20 s between each series of stimuli. T1 calibration was performed, and recordings of TOF stimulation were obtained while the patient was awake. The following data were recorded after drug administration: (1) onset time of mivacurium (time from completion of drug administration to maximum inhibition at the threshold), non-response time, recovery index (RI) (time for T4/T1 to recover from 25% to 75%), (2) supplementary doses of mivacurium, (3) vocal cord movements observed during the operation, and (4) adverse reactions such as skin rash, bronchospasm, and arrhythmia.
Statistical analysis
A statistical analysis was conducted using IBM SPSS 25.0 and GraphPad Prism 9.0. For normally distributed measurements, data were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was employed to compare multiple groups. When the assumption of normal distribution was not met, the rank sum test was used, and the results were expressed as the me-dian with the 25th and 75th percentiles: M (P25, P75). The t-test was utilized to compare two groups, with corrections made when the variances were non-homogeneous. Enumeration data were presented as percentages. The chi-square (c2) test was used for comparisons between multiple groups. Statistical significance in the screening difference was the independent variable while the dependent variable was drug supplementation. For further binary logistic regression analysis, the receiver operating characteristic (ROC) method was employed to determine the optimal cut-off value when the independent variable was continuous. Pearson’s correlation coefficient was used to examine the relationship between the total amount of medication and the recovery index, age and the intensity of the stimulation. Multiple linear regression models were employed to identify the factors that influence the recovery indexes. A significance level of P < 0.05 was considered statistically significant.
RESULTS
There were no statistically significant differences in age, weight, height, BMI or comorbidities among the three groups (Table 1). During the intubation process, there was clear visualization of the vocal cords and minimal patient discomfort. All patients were successfully intubated, and no body movements were observed during the procedure. During the induction process, 3 patients experienced transient redness of the skin at the injection site (arm) with an incidence rate of 5%. There were no instances of airway spasms or significant fluctuations in blood pressure or heart rate. After the surgical procedure, the patients regained consciousness and demonstrated good respiratory functions.
TABLE 1
Demographic characteristics of the three patient groups
Neuromuscular monitoring
The stimulus current intensity during T1 calibration was 33.86 ± 4.15 mA for male patients and 33.00 ± 5.20 mA for female patients. This difference was not statistically significant (P > 0.05; Table 2). Furthermore, no statistically significant correlation was found between age and the intensity of stimulation currents (P = 0.266).
Pharmacodynamic indicators of mivacurium
During the anaesthesia induction process, the mean onset time of mivacurium target-controlled infusion at a dose of 0.2 mg kg–1 was 4.37 ± 0.83 minutes. There was no statistically significant difference in the onset time of mivacurium among the three groups of patients. The mean no-response time was 3.94 ± 1.01 minutes and was not statistically significantly different among the three groups. Fifteen patients (75%) in group C1, 10 patients (50%) in group C2, and 3 patients (15%) in group C3 required the administration of additional mivacurium during surgery. The differences between group C3 and group C1, as well as between group C3 and group C2, were statistically significant (P < 0.05; Table 3). In group C1, when T1 reached 0, two patients (10%) experienced vocal cord immobility during the surgery, whereas no patients in groups C2 and C3 exhibited this symptom. However, there was no statistically significant difference between group C1 and group C2 (P > 0.05; Table 3). TOF75% and TOF90% were not significantly different between the three patient groups. The mean RI for the three groups of patients was 7.29 ± 2.54 minutes, and the difference among the three groups was not statistically significant (P > 0.05; Table 3).
TABLE 3
Pharmacodynamics of mivacurium in the three patient groups
* denotes a P-value < 0.05, the difference between the groups was statistically significant; a indicates a P-value < 0.05, the difference was statistically significant when compared to group C1 and group C2. For pairwise comparisons, χ2 segmentation was calculated with a significance level adjusted to α = 0.0125.
The analysis of independent variables using univariate and multivariate binary logistic regression showed that RI had no relationship with age, gender, BMI, onset of muscle relaxation, or no-response time. However, an RI greater than 5.68 was correlated with a reduced need for additional intraoperative mivacurium dosage (P < 0.05; Table 4).
TABLE 4
Univariate and multivariate binary logistic regression results of independent variables
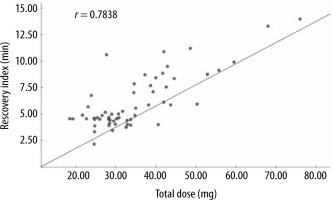
When RI was set as the dependent variable in multiple linear regression, it was not linearly correlated with age, sex, BMI, or muscle relaxation onset time, but it was linearly correlated with no-response time and the total dose of mivacurium (F = 19.585, P < 0.001, R2 = 0.689; Table 5). Pearson’s correlation analysis revealed a significant positive correlation (r = 0.7838) between the RI and total mivacurium dosage in all patient groups (P < 0.001, 95% CI: 0.6617–0.8655; Figure 2).
TABLE 5
Multiple linear regression with recovery index as the dependent variable
DISCUSSION
In this study, there were no statistically significant differences in patient demographics or adverse reactions during anaesthesia induction. Only 5% of patients exhibited transient skin redness at the injection site during the induction process. Neuromuscular monitoring revealed that the intensity of stimulation remained consistent across genders and was not correlated with age. With higher mivacurium infusion dosage, a shorter muscle relaxa-tion onset time, longer no-response time, quicker offset speed of neuromuscular block and slower complete recovery of neuromuscular function were observed. However, these factors were not statistically significantly different among the 3 groups. It is noteworthy that group C1 required a significantly larger amount of additional mivacurium during the surgery. In addition, 10% of patients in group C1 experienced vocal cord immobility when T1 reached 0, although this difference was not statistically significant. Age, gender, BMI, and onset of muscle relaxation did not significantly influence the RI. An RI exceeding 5.68 minutes had a protective effect against the need for additional mivacurium administration to maintain muscle relaxation. Moreover, RI was significantly associated with no-response time and the total dose of mivacurium administration. In addition, higher mivacurium dosages were linked to faster muscle recovery.
Mivacurium is characterized by its distinctive chemical structure, which combines the bisbenzyltetrahydroisoquinoline framework of atracurium with an ester linkage in succinylcholine. This composition makes mivacurium susceptible to enzymatic degradation, resulting in certain drawbacks that are associated with both of its structural components. These include a delayed onset of action and a tendency to induce allergic reactions [6]. In the present study, we administered 0.2 mg kg–1 of mivacurium for anaesthesia induction, with an average onset time of 4.48 ± 1.03 minutes to achieve adequate muscle relaxation for endotracheal tube placement. As a result, all 60 patients achieved favourable intubation conditions and underwent successful tracheal intubation. A meta-analysis revealed that higher induction doses of mivacurium and prolonged time to intubation were linked to a greater probability of achieving optimal intubation conditions [32]. However, while an induction dose of 0.25 to 0.3 mg kg–1 can reduce the onset time to 1–1.5 minutes, it carries the risk of hypotension due to histamine release [12]. On the other hand, a delayed intubation time following mivacurium induction enables a more potent effect on muscle relaxation by increasing its accumulation at the neuromuscular junction, which intensifies its inhibitory effect [32].
In this study, only 3 patients experienced transient skin redness at the injection site during induction, which might be attributable to histamine release, local irritation, or allergic sensitivity. However, no cases of facial flushing, reflex tachycardia, or hypotension were reported. Histamine release is a significant adverse effect associated with mivacurium, potentially leading to unstable haemodynamics and thereby restricting its practical utility [33]. Neuromuscular blocking agents are typically associated with type 1 hypersensitivity reactions, also known as immediate hypersensitivity. These reactions are characterized by the rapid release of proteases, including tryptase, preformed histamine and newly synthesized vasoactive mediators, from basophils and mast cells triggered by cross-linking of muscle relaxants with membrane-bound IgE [34]. The release of these mediators can lead to skin reactions, bronchospasm and cardiovascular symptoms [35]. It was previously reported that mivacurium induced histamine release when administered at high doses of 0.20 mg kg−1 or more, equivalent to more than three times the ED95, and with rapid administered in less than 30 seconds [36]. Therefore, a target-controlled infusion of mivacurium, characterized by a controlled and gradual delivery, might mitigate histamine release and minimize the risk of allergic reactions compared to rapid bolus injections, which involve the administration of larger doses at a faster rate.
Mivacurium has a short half-life and rapid clearance due to a rapid enzymatic hydrolysis by butyrylcholinesterase, leading to a relatively short duration of neuromuscular blockade [6, 37]. Since the duration of laser laryngeal microsurgery for vocal tumours typically ranges from 30 to 60 minutes, the rapid clearance of mivacurium provides the basis for the continuous infusion of mivacurium during the procedure. It was previously reported that the mean consumption of mivacurium for continuous infusion in adults to sustain neuromuscular blockade at 89–99% twitch suppression typically falls in the range of 6–7 g kg–1 min–1 without employing a control system [38]. Janda et al. observed that a mivacurium dosage of 4.25 ± 1.25 μg kg–1 min–1 was sufficient to sustain a 90% neuromuscular blockade [39]. Conversely, Kansanaho et al. [40] demonstrated that an average mivacurium consumption of 7.5 ± 3.1 μg kg–1 min–1 was associated with 95% neuromuscular blockage while Schumacher et al. reported that a consumption rate of 7.0 ± 2.2 μg kg–1 min–1 was linked to a neuromuscular blockage of 90% [21], which might potentially be influenced by variations in patients and surgical procedures. In order to ensure deep muscle relaxa-tion during laser-assisted laryngeal microsurgery, the maintenance dose of mivacurium in this study was set to maintain a 100% neuromuscular blockade with T1 at 1% for supplementary dosage, thus effectively preventing laryngeal reflex and autonomous vocal cord movement.
When mivacurium was administered as a continuous infusion at a rate of 6 μg kg–1 min–1, 2 patients experienced involuntary vocal cord movement during the procedure and 15 patients required additional mivacurium doses to maintain muscle relaxation, indicating insufficient neuromuscular blockade at this low dose, which might potentially impact the accuracy of laser surgery. In group C2, which received 7 μg kg–1 min–1, 10 patients needed additional doses. In contrast, patients in group C3, who received 8 μg kg–1 min–1, displayed no autonomous vocal cord movements during the surgery, required supplementary doses, and had no significant blood pressure or heart rate fluctuations, ultimately achieving optimal surgical conditions. In line with our results, Yongjie et al. [41] applied a sequential method to establish the maximum safe dose of mivacurium for continuous infusion in thyroid surgery, which was found to be 8.94 μg kg–1 min–1, with a 95% confidence interval ranging from 8.89 to 8.99 μg kg–1 min–1. These findings suggest that precise mivacurium dosing is vital for achieving optimal conditions in laser surgery, with a dosage of 8 μg kg–1 min–1 achieving the most favourable outcomes.
In the present study, individual stimulation current calibration was performed in order to ensure precise neuromuscular monitoring. A prior study demonstrated that maintaining the stimulation current at least 15 mA above the threshold resulted in variances of up to 10% in individual TOF ratios [42], indicating the importance of calibrating stimulation currents. In addition, it was found that the postoperative effects of neuromuscular blocking drugs affected male patients more significantly than female patients, further emphasizing the need for tailored calibration [42, 43]. Nonetheless, it remains unclear whether males have a higher incidence of complications related to neuromuscular blocking drugs compared to females [44]. Taking individual differences into account, we conducted T1 calibration before anaesthesia induction for all participants. Our findings revealed no significant differences in the stimulation current values during T1 calibration between male and female patients. Furthermore, we did not observe a relationship between the RI and gender.
When the RI exceeded 5.68 minutes, additional intraoperative doses of mivacurium were not warranted. A longer RI, indicating a slower recovery of neuromuscular function, suggests that mivacurium remains effective for an extended duration, reducing the need for supplementary doses during the surgery to maintain the desired level of muscle relaxation. It was demonstrated that the prolonged infusion of mivacurium did not lead to significant accumulative effects, and the recovery time was independent of the total administered dose [38]. In contrast, our findings revealed a positive correlation between the RI and the total administered dose, signifying that the recovery time increased with higher doses of mivacurium. Various factors, such as genetic mutations in the butyrylcholinesterase gene, haemodilution, impaired hepatic function, albumin loss, malnutrition, pregnancy and oestrogenic effects, could prolong the effects of mivacurium by a few minutes to several hours [45–48]. Additionally, a previous investigation found that the cis–trans and trans–trans isomers were rapidly eliminated from the body within approximately 2 minutes, while the cis–cis isomer had a longer elimination time of about 52 minutes [49]. An accumulation of the cis–cis isomer in the plasma could contribute to a prolonged recovery period and increase the likelihood of residual neuromuscular blockade after the surgery [15, 50].
LIMITATIONS
There are several limitations in this study that should be acknowledged. While the objective of our study was to determine the optimal mivacurium infusion dosage for laryngomicroscopic surgery using a target-controlled infusion system, we did not conduct a comprehensive comparative analysis of mivacurium’s effects and recovery compared to other neuromuscular blocking agents, such as rocuronium and atracurium. In addition, 3 patients among those who received a continuous mivacurium infusion at 8 μg kg–1 min–1 required supplemental mivacurium doses to maintain deep muscle relaxation, emphasizing the importance of individualized monitoring and dosing strategies. Furthermore, this study was conducted at a single centre and involved a relatively small sample size. Further investigations with larger patient populations across many centres are needed in order to further explore the pharmacodynamics of mivacurium and corroborate the findings of the current paper.
CONCLUSIONS
This study investigated the effects of different doses of mivacurium in patients undergoing laser-assisted laryngeal microsurgery for vocal cord tumours. A target-controlled infusion of mivacurium at a dose of 0.20 mg kg–1 achieved optimal intubation conditions during anaesthesia induction while minimizing allergic reactions. Such allergic reactions, particularly histamine release, can pose significant challenges in maintaining stable haemodynamics during induction. Therefore, the controlled and gradual administration of mivacurium emerges as a safer and more efficacious approach. Moreover, the continuous infusion of mivacurium at a rate of 8 μg kg–1 min–1 stood out as the most suitable maintenance strategy, ensuring a favourable surgical environment by preventing involuntary vocal cord movement, bronchospasm, or significant haemodynamic fluctuations, and minimizing the need for supplementary doses. This dosage regimen has the potential to enhance patient safety and ensure smoother surgical procedures, particularly in delicate settings such as laryngeal microsurgery. In addition, this study provided insights into factors influencing the RI, including no-response time and the total dose of mivacurium, and revealed a positive correlation between the RI and the total administered dose of mivacurium. An increase in the total drug dosage during continuous mivacurium infusion might lead to delayed recovery of muscle relaxation and residual neuromuscular blockade and thus necessitates careful management. These findings might enable anaesthesiologists to make informed decisions regarding mivacurium dosage and better manage patients undergoing laryngeal microsurgery. Overall, the results underscore the significance of tailored approaches to neuromuscular blockade management and contribute to our understanding of mivacurium’s pharmacodynamics, helping to enhance patient safety and surgical outcomes. Further research and clinical applications of these findings hold the potential to improve the quality of care in anaesthesia and laryngeal microsurgery.